Elise Reuter Reporter

FURKAN TELLIOGLU via Getty Images
Dive Brief:
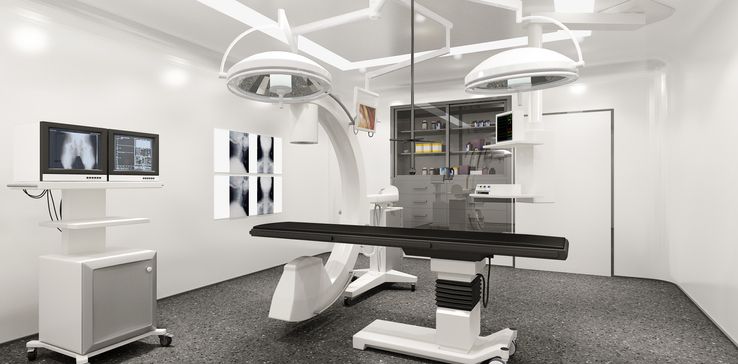
- GE Healthcare and Medtronic said on Thursday they agreed to a partnership focused on outpatient care in which both will provide medical device products and software to ambulatory surgery centers (ASCs) and office-based labs (OBLs).
- GE Healthcare will provide support with planning, construction and imaging equipment, while Medtronic plans to provide devices for cardiac rhythm, pain management, peripheral vascular and spinal compression fracture procedures, as well as support with reimbursement.
- The collaboration comes as more procedures are shifting to outpatient care centers, driven in part by changes to reimbursement and limitations in hospital capacity during the pandemic.
Dive Insight:
Although GE Healthcare and Medtronic will still promote their products separately, it will be done in a collaborative manner with the goal of making it easier for owners of ambulatory surgery centers and office-based labs to execute on their projects, according to an emailed statement from James Rapp, GE Healthcare’s vice president of interventional imaging.
The partnership is intended to help providers navigate the cost and complexity of building a new ambulatory surgery center or expanding an existing one.
“As our customers open centers outside of the hospital, they are looking for support beyond the devices used in medical procedures,” Adam King, Medtronic’s senior director of U.S. enterprise accounts and ambulatory surgery centers, said in Thursday’s announcement. “From products and devices to equipment and services, we provide a full range of technologies and solutions. Our collaboration with GE Healthcare was formed to better serve the growth of our ASC and OBL customers with extensive technologies and dedicated teams who have expertise in outpatient services and can address all aspects of this evolving sector.”
The two companies pointed to growth in outpatient procedures as part of the reasoning behind the partnership. After the start of the COVID-19 pandemic as hospitals grappled with limited capacity and staffing shortages, more procedures shifted into the outpatient environment.
Reimbursement changes also have been a contributing factor. In 2020, the CMS approved payments for certain cardiovascular procedures, leading to fixed c-arm systems being used more frequently for outpatient cardiac and peripheral vascular procedures.
Boston Scientific also pointed to a report from Research and Markets showing that the market for ambulatory surgical centers is expected to expand at a compound annual growth rate of 7.5% from 2019 to 2029.
Other sectors also are closely watching the shift to outpatient care. For instance, orthopaedics companies such as Stryker, Zimmer Biomet, and Johnson & Johnson’s DePuy Synthes also have discussed the opportunity as the pandemic has accelerated the shift to ambulatory surgery centers.
RBC Capital Markets Analyst Shagun Singh said in an April 19 research note that procedure volumes have started to recover, especially in higher acuity procedures.
“We favor companies with care settings exposure to [ambulatory surgery centers] or [hospital-based outpatient departments] as opposed to elective procedures in the near-term, as the former have less acute staffing shortages,” Singh wrote.
However, even lower-acuity procedures are starting to see a recovery as well, such as hips and knees, and some diagnostic and surgical procedures.
– Elise Reuter @EliseReuter
Source: MedTech Dive
-
In accordance with Medical Device Authority (MDA Malaysia Ministry of Health, Malaysia), MyMedicNews serves as the leading online media in the medical device industry for Malaysia and the ASEAN region. We provide a creative one-stop web portal to deliver the latest news and product development in the medical device industry. Through our portal, key industry players can deliver business information, product, and services effectively to relevant target audience.
Disclaimer: All content is for informational or educational purposes only, and does not substitute professional medical advice or consultations with healthcare professionals. Medical device advertisements and their content are intended for Healthcare Professionals only. More information related to Malaysia medical device news, products, registration, and regulations is available on Medical Device Authority.