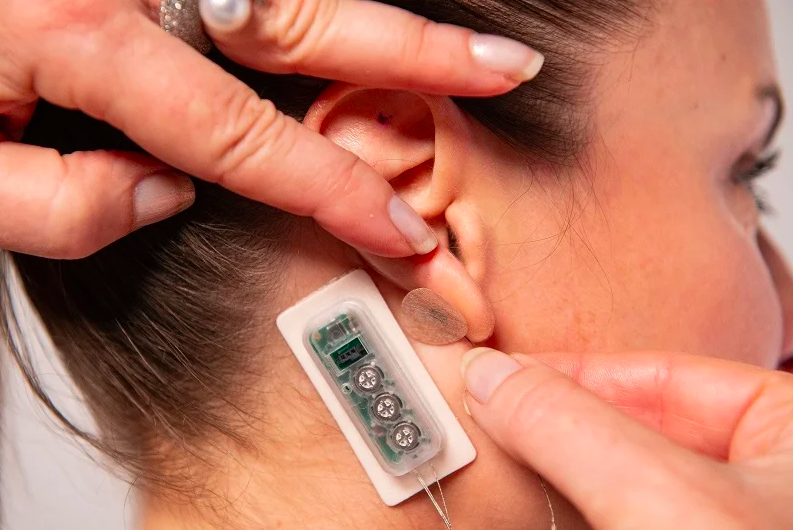
DyAnsys has received US Food and Drug Administration (FDA) approval for its percutaneous electrical neurostimulation (PENS) device, called First Relief, to treat diabetic neuropathic pain.
First Relief is intended for symptomatic relief of chronic, intractable pain related to diabetic peripheral neuropathy through multiple treatments for up to 56 days.
Placed behind the ear, the wearable device provides continuous pulses of low-level electrical current for several days.
DyAnsys CEO Srini Nageshwar said: “We are excited to have the FDA clearance of First Relief so that this device, which has been proven effective, can now be used to treat patients who have been experiencing pain related to diabetic neuropathy.
“First Relief offers a significant treatment option without drugs or narcotics.”
The regulatory approval was based on data obtained from a randomised, double-blind, single-centre, controlled prospective study, which was conducted with 63 participants aged between 30 and 74 years.
In the study, First Relief was evaluated against a placebo, as well as another device that had previously been approved by the US FDA.
The devices were applied for a period of 16 weeks on a bi-weekly basis.
Pain intensity was measured through the Visual Analog Scale (VAS) score as the study’s primary efficacy endpoint.
The insomnia severity index (ISI), vibration perception threshold (VPT) value, total neuropathy limitations scale (ONLS) and Hamilton rating scale for anxiety were measured as some of the study’s secondary endpoints.
Based on the findings from the study, subjects treated with First Relief experienced a significant reduction in VAS pain score from the start of treatment to the end, with the improvements continuing throughout 90 days of follow-up.
This suggests that the device can provide long-term improvement in neuropathic pain.
DyAnsys noted that similar improvements were also observed for all secondary endpoints, demonstrating significant improvement in sleep and mood alongside a decrease in neuropathic pain.
Source: medicaldevicenetwork
-
In accordance with Medical Device Authority (MDA Malaysia Ministry of Health, Malaysia), MyMedicNews serves as the leading online media in the medical device industry for Malaysia and the ASEAN region. We provide a creative one-stop web portal to deliver the latest news and product development in the medical device industry. Through our portal, key industry players can deliver business information, product, and services effectively to relevant target audience.
Disclaimer: All content is for informational or educational purposes only, and does not substitute professional medical advice or consultations with healthcare professionals. Medical device advertisements and their content are intended for Healthcare Professionals only. More information related to Malaysia medical device news, products, registration, and regulations is available on Medical Device Authority