Asensus Surgical, a US-based medical device company that is digitising the interface between the surgeon and the patient to pioneer a new era of Performance-Guided Surgery, has announced that Kitakyushu General Hospital in Japan has entered into an agreement to lease and utilise a second Senhance Surgical System. Kitakyushu is the first hospital to install multiple Senhance Surgical Systems.
The hospital previously initiated their first Senhance system in January of 2020, and since has completed over 300 procedures utilizing Performance-Guided Surgery.
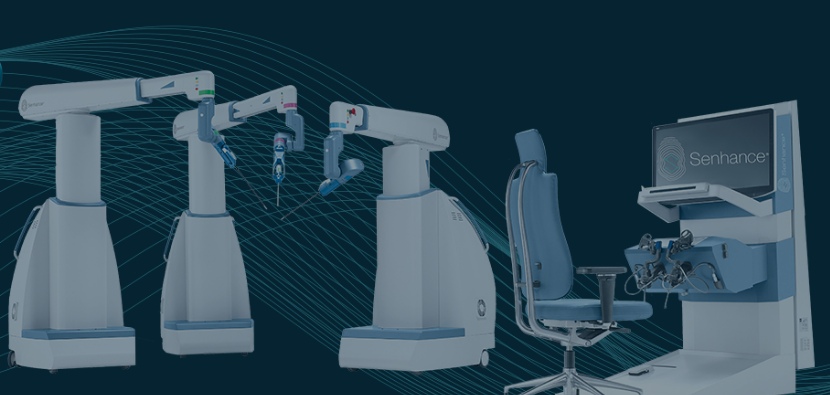
Asensus Surgical's technology platform, the Senhance Surgical System, is the first of its kind digital laparoscopic platform that leverages augmented intelligence to provide unmatched performance and patient outcomes through machine learning. Senhance goes beyond the typical surgical robotic systems, providing surgical assurance through haptic feedback, eye-tracking camera control, and 3D visualization, and is the first platform to offer 3mm instruments (the smallest instrument available in the world on a robotic surgical platform).
The Senhance Surgical System is powered by the Intelligent Surgical Unit (ISU). The ISU enables machine vision-driven control of the camera for a surgeon by responding to commands and recognising certain objects and locations in the surgical field, and allows a surgeon to change the visualised field of view using the movement of their instruments.
Source: BioSpectrumAsia
-
In accordance with Medical Device Authority (MDA Malaysia Ministry of Health, Malaysia), MyMedicNews serves as the leading online media in the medical device industry for Malaysia and the ASEAN region. We provide a creative one-stop web portal to deliver the latest news and product development in the medical device industry. Through our portal, key industry players can deliver business information, product, and services effectively to relevant target audience.
Disclaimer: All content is for informational or educational purposes only, and does not substitute professional medical advice or consultations with healthcare professionals. Medical device advertisements and their content are intended for Healthcare Professionals only. More information related to Malaysia medical device news, products, registration, and regulations is available on Medical Device Authority