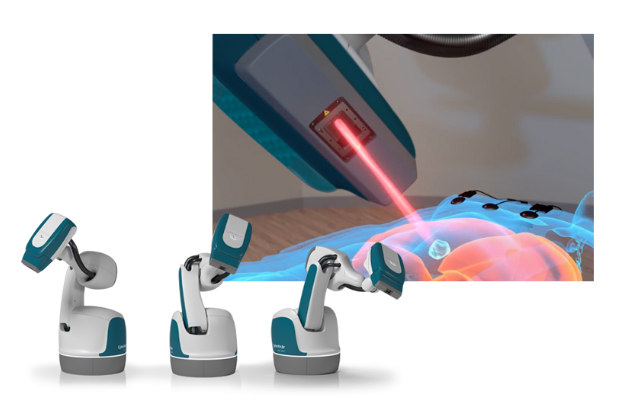
US-based biomedical firm Accuray has announced that Auckland Radiation Oncology (ARO) is the first centre in New Zealand to treat patients with the CyberKnife System, a robotic radiation therapy device known for delivering treatments with sub-millimeter precision and accuracy in typically 1 to 5 outpatient sessions.
The ARO medical care team selected the latest generation CyberKnife S7 System to provide stereotactic radiosurgery (SRS) and stereotactic body radiation therapy (SBRT) to patients more efficiently, minimising the time they spend in daily treatments and maximizing the number of New Zealand cancer patients they can treat each day.
"These first-in-country patient treatments reinforce the value the CyberKnife System provides to medical care teams around the globe. The addition of the Auckland Radiation Oncology centre to the network of facilities using the CyberKnife System will broaden access to a trusted radiation therapy treatment option," said Suzanne Winter, President and CEO of Accuray.
The CyberKnife S7 System combines speed, precision and motion synchronisation technology for the treatment of lesions and tumors throughout the body. Treatment is non-surgical, non-invasive and does not require incisions or general anesthesia. Clinical studies support the use of the CyberKnife System, expanding medical care team options for achieving outstanding outcomes for a wide range of indications.
Source: BioSpectrumAsia
-
In accordance with Medical Device Authority (MDA Malaysia Ministry of Health, Malaysia), MyMedicNews serves as the leading online media in the medical device industry for Malaysia and the ASEAN region. We provide a creative one-stop web portal to deliver the latest news and product development in the medical device industry. Through our portal, key industry players can deliver business information, product, and services effectively to relevant target audience.
Disclaimer: All content is for informational or educational purposes only, and does not substitute professional medical advice or consultations with healthcare professionals. Medical device advertisements and their content are intended for Healthcare Professionals only. More information related to Malaysia medical device news, products, registration, and regulations is available on Medical Device Authority