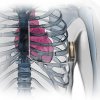
Boston Scientific received approval in the U.S. and Europe for the second generation of its subcutaneous implantable defibrillator (S-ICD). The EMBLEM S-ICD System, originally developed by Cameron Health, a company acquired by Boston Scientific, provides protection against sudden cardiac arrest, but without heart leads used with all other implantable defibrillators.
Since the system delivers electric shocks through a lead placed just under the skin next to the sternum, there’s no need to run wires into the heart and keep them attached to the moving organ. This is a significant advantage during implantation and avoids post-op complications that are associated with leads that have been known to detach.
The new EMBLEM S-ICD is 19% thinner and has a battery life 40% longer than the original device. This means that it will be more comfortable to wear and less noticeable while requiring fewer replacements. Moreover, the system woks with the company’s LATITUDE NXT Patient Management System, helping cardiologists to keep track of their patients.
source:Boston Scientific
Emblem Second Generation Subcutaneous Defibrillator