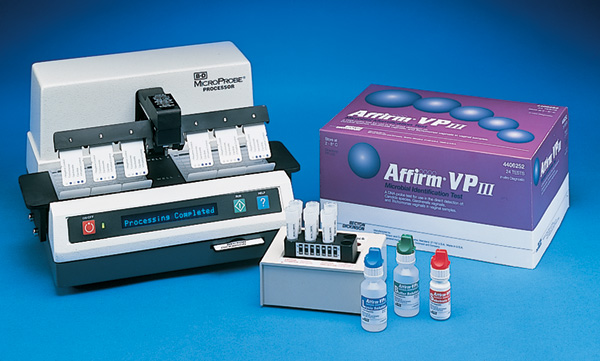
The Affirm™ VPIII Microbial Identification Test is the first direct specimen RNA probe-based diagnostic test for the differential detection and identification of the causative agents for vaginitis: Candida species, Gardnerella vaginalis and Trichomonas vaginalis.

The Affirm™ VPIII test’s RNA probe technology offers a dependable, rapid means toward early identification and treatment of patients suffering from these pathogens. The test features an easy-to-read visible color reaction that is more accurate than current microscopic methods for detecting the causative agents of vaginitis. Additionally, its performance is unhampered by "difficult specimens," self-medication or the presence of mixed infections.
During the test process, the assay aligns complementary nucleic acid strands to form specific, double-stranded complexes called hybrids. For each organism, this hybrid is composed of capture and color development single-stranded nucleic acid probes, complementary to the released target nucleic acid analyte. Enzyme conjugate then binds to the captured analyte.
A positive result is a visible blue color reaction on the organism-specific bead embedded in the Affirm™ Probe Analysis Card (PAC). Each PAC contains three vaginitis organism test beads, plus positive and negative control beads to ensure test quality.
Total time-to-results is under 45 minutes, with less than 2 minutes hands-on time needed. The simple, automated procedure can be performed with minimal training.
System components include the BD MicroProbe™ Processor, Lysis Block, Affirm™ VPIII Sample Collection Set and Affirm™ VPIII Test Kit. Sufficient reagents and materials are supplied in each test kit to perform 24 patient tests.