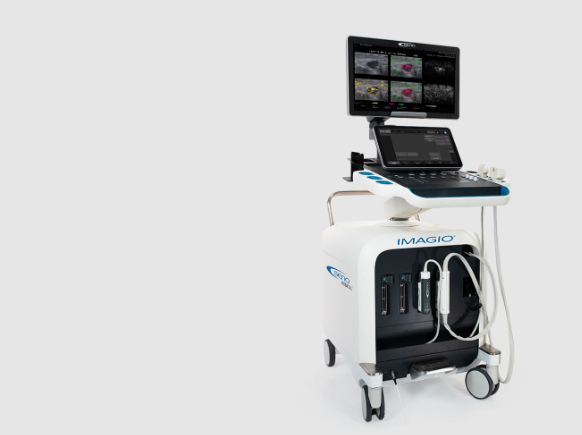
San Antonio, TX, Jan. 13, 2023 (GLOBE NEWSWIRE) -- Seno Medical has entered into an exclusive distributor agreement with Genetik, Inc., to market, sell and service the company’s groundbreaking Imagio® Opto-Acoustic/Ultrasound (OA/US) Breast Imaging System. This distributor agreement – a first for Seno – will enable Genetik to regionally distribute the Imagio® System in Singapore, where the distributor is headquartered.
“We are thrilled to be partnering with a reputable organization such as Genetik to stimulate commercial adoption of the Imagio® System into Southeast Asia,” commented Tom Umbel, CEO and President of Seno Medical. “This agreement represents a major milestone for Seno Medical and the continued acceptance and adoption of our new fusion modality in breast imaging.”
“We are proud to work with innovative and highly accurate technologies that can change the standard of care for patients. We look forward to representing this state-of-the-art product for diagnosing breast cancer – the only global FDA-approved technology based on photoacoustic fused with ultrasound,” commented Genetik CEO V. B. Balrai Singh.
Recently honored with a Gold Edison award, a Gold Medical Design Excellence Award and as a semi-finalist in the 2022 Minnie’s for its medical innovation, Seno’s groundbreaking diagnostic breast cancer imaging system helps physicians differentiate between benign and malignant breast lesions using non-invasive opto-acoustic/ultrasound (OA/US) technology to provide information on breast lesions in real time, helping providers to characterize masses that may — or may not — require more invasive diagnostic evaluation.
Breast biopsy procedures caused by false-positive diagnostic assessments cost the US healthcare system more than $2 billion per year.[i] Seno’s Imagio® technology could significantly reduce those costs with its patient-centric OA/US innovation.
Seno’s OA/US system combines laser optics and grayscale ultrasound to provide fused functional, morphological, and anatomical breast imaging. The opto-acoustic images provide a unique blood map in and around breast masses, while the ultrasound provides a traditional anatomical image. Through the appearance or absence of two hallmark indicators of cancer — angiogenesis and hypoxia — Seno Medical has shown that the Imagio® OA/US Breast Imaging System will be a more effective tool to help radiologists confirm or rule out malignancy compared with traditional diagnostic imaging modalities. And it does this without exposing patients to potentially harmful ionizing radiation (x-rays) or contrast agents. In addition to the novel imaging provided by the Imagio® System, Seno includes an artificial intelligence (AI) decision-support tool (SenoGram®) to help physicians interpret the new images. This AI tool, along with training and certification, helps radiologists transition from ultrasound alone to OA/US imaging to more precisely assign the likelihood of malignancy.
The Imagio® System was cleared by the US FDA in 2021 and received a supplemental PMA approval for its market-ready device in June 2022. The system is indicated for use by trained and qualified healthcare providers to evaluate palpable and non-palpable breast abnormalities in adult patients who are referred for diagnostic imaging breast work-up following clinical presentation or other imaging examinations such as screening mammography.
Seno Medical Instruments, Inc. is a San Antonio, Texas-based medical imaging company committed to developing and commercializing a new modality in cancer diagnosis: opto-acoustic imaging. Originally approved by the US FDA in January 2021 with a Supplemental PMA Approval for its latest device in June 2022, Seno Medical’s Imagio® Breast Imaging System fuses opto-acoustic technology with ultrasound (OA/US) to generate real-time functional and anatomical images of the breast. To learn more about Seno Medical’s OA/US imaging technology and applications, visit www.SenoMedical.com.
Source: BioSpace
-
In accordance with Medical Device Authority (MDA Malaysia Ministry of Health, Malaysia), MyMedicNews serves as the leading online media in the medical device industry for Malaysia and the ASEAN region. We provide a creative one-stop web portal to deliver the latest news and product development in the medical device industry. Through our portal, key industry players can deliver business information, product, and services effectively to relevant target audience.
Disclaimer: All content is for informational or educational purposes only, and does not substitute professional medical advice or consultations with healthcare professionals. Medical device advertisements and their content are intended for Healthcare Professionals only. More information related to Malaysia medical device news, products, registration, and regulations is available on Medical Device Authority